Abstract
Introduction When treating Hodgkin lymphoma (HL), it is common UK practice to modify escalated BEACOPP (eBPP) by removing oral procarbazine and replacing it with intravenous dacarbazine (250mg/m2 D2-3) to reduce haematopoietic stem cell and gonadal toxicity. A similar replacement of procarbazine in COPP (to COPDac) has reduced gonadal toxicity and conferred a comparable long-term event-free survival to children (EuroNet-PHL-C1 trial; Mauz-Körholz et al Lancet Oncol 2021). Our own retrospective study has shown that adults treated with first-line escalated BEACOPDac (eBPDac) have a reduced blood transfusion requirement and earlier return of menstrual periods compared with real-world eBPP patients (pts) with no loss of clinical efficacy compared to HD18 eBPP pts (Santarsieri et al Blood 2021). To better understand the toxicity differences, we performed this study to compare the haematopoietic stem and progenitor cell (HSPC) mutational burden in pts who have received procarbazine and dacarbazine containing regimens. Chemotherapeutic agents are known to damage DNA and this mutational process likely causes many of the long-term chemotherapy toxicities. Little is known about the mutational signatures and mutation burdens caused by HL therapies, although a novel mutational signature (SBS25) has recently been described in two HL cell lines (Petljak et al Cell 2019).
Methods We whole genome sequenced 6-8 single-cell derived HSPC colonies from each of 12 advanced stage HL pts (n = 91; mean sequencing depth 26X). The pts were all in remission > 6 months and had been previously treated with either eBPDac (6 cycles (n=2); 4 cycles (n=2)), eBPP (6 cycles (n=3); 5 cycles (n=1); 4 cycles (n=1)) or ABVD (n=3; all 6 cycles). Single HSPC somatic mutation burdens and mutation spectra from these chemotherapy exposed individuals were compared to those from our previously published normal cohort (n = 110; mean sequencing depth 24X) (Mitchell et al Nature 2022). In addition we have updated the outcomes of our retrospective multi-centre study of pts treated with eBPDac including additional pts and longer follow-up of the entire cohort.
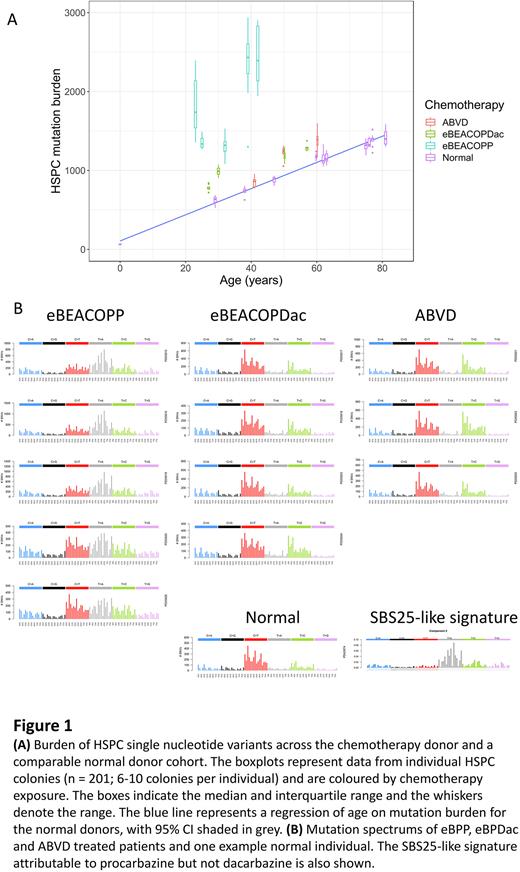
Mutational analysis The somatic single nucleotide variant mutation burden in normal adult HSPCs ranges from approximately 400 aged 20yr to 1500 aged 80yr; an average of 18 mutations accumulate with every year (Figure 1A). The HSPCs from ABVD and eBPDac-treated pts had similar minor excess somatic mutation burdens compared to aged-matched normal HSPCs of 183 (CI95%= 110-256) and 291 (CI95%= 242-340) mutations respectively. In contrast, the HSPCs from eBPP-treated pts had a striking and significantly increased excess mutation burden of 1153 (CI95%= 937-1369). Of the eBPP pts, the single patient who received 4 cycles had the lowest number of excess mutations, but noting the inter-patient variation, we cannot yet conclude that eBPP cycle number correlates with the number of mutations. Descriptive analysis of the mutational profiles (Figure 1B) revealed that every patient who received procarbazine had a clear SBS25-like mutational signature, characterised by T>A base substitutions, demonstrating for the first time that this DNA mutational signature is directly attributable to procarbazine.
Clinical data analysis The clinical data presented last year have been updated with more pts and longer follow-up (n=288; median 2.3 yr). With this high-risk group of advanced stage pts, our data suggest that the eBPDac pts have reduced red cell transfusion requirements, reduced hospitalisation, improved menstrual period recovery and improved sperm count preservation compared with eBPP pts. The eBPDac 24-month PFS is similar to HD18 3-yr PFS (94.9% vs 92.3%) and appears superior to RATHL 5-yr PFS (81.4%). The eBPDac 24-month OS estimate is 99%.
Conclusions We have shown that dacarbazine-containing regimens (ABVD and eBPDac) confer a lower mutation burden to HSPCs compared with procarbazine-containing eBPP and that procarbazine is the agent responsible for the SBS25 mutational signature. This work provides a model example of how genomic analysis could be used to inform therapeutic decision making in cancer. Our retrospective analysis provides strong supportive evidence that eBPDac is a highly efficacious HL treatment and noting the clear benefits in terms of HSPC genomic health, we would encourage clinicians offering eBPP to HL pts to consider replacing procarbazine with dacarbazine.
Disclosures
Santarsieri:Janssen: Honoraria; Takeda: Other: Conference funding 2021. Osborne:MSD: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Roche: Honoraria; Servier: Honoraria; Gilead: Honoraria. Ardeshna:Novartis: Honoraria; Gilead: Honoraria; BMS: Honoraria. Collins:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau. Cwynarski:Roche, Takeda, KITE/Gilead, Incyte: Speakers Bureau; Roche, Takeda, Celgene/BMS, Atara, Gilead/KITE, Janssen, Incyte: Consultancy; Roche, Celgene/BMS, Takeda, KITE: Other: Travel to scientific congress; BeiGene: Research Funding. Davies:Kite, a Gilead company: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; GSK: Other: Research support; Ascerta Pharma/Astra Zeneca: Honoraria, Other: Research support; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support. Travel to scientific conferences, Research Funding; Celegne (a Bristol Myers Squibb company): Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences, Research Funding. Furtado:Abbvie: Other: Conference support. Gallop-Evans:Kyowa-Kirin: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Educational Support. Iyengar:AbbVie: Other: conference support; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: conference support, Speakers Bureau; Lilly: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau. Linton:BMS/Celgene: Consultancy; Kite/Gilead: Consultancy; Beigene: Consultancy; Roche: Consultancy; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Martinez-Calle:AstraZeneca: Honoraria, Other: travel support; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Other: travel support; Janssen: Honoraria. McKay:Abbvie: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Celgene/BMS: Consultancy; Epizyme: Consultancy; Gilead/Kite: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Recordati Rare Diseases: Consultancy; Roche: Consultancy; Takeda: Consultancy. Nagumantry:Alexion: Honoraria; Janssen: Honoraria. Shah:Abbvie: Consultancy; Janssen: Consultancy. Uttenthal:Takeda: Honoraria; Roche: Honoraria; Jazz: Honoraria. McMillan:Prosethetics: Honoraria; Amgen: Honoraria; Roche: Honoraria; Takeda: Honoraria, Other: Travel funding. Laurenti:GlaxoSmithKline: Research Funding. Campbell:FL86 Inc: Consultancy, Other: Founder. Follows:Lilly: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Abbvie: Honoraria; Roche: Honoraria; Astra Zeneca: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.